Diamond (C)
by
Julian Gray, P.G.

Name: Known from antiquity. The name is a corruption of the Greek word, adamas, which means invincible; probably due to its hardness (Blackburn and Dennen, 1997).
Varieties: Ballas, bort, carbonados
Mineral Class: Native element. Diamond is a polymorph of chaoite, graphite, and lonsdaleite.
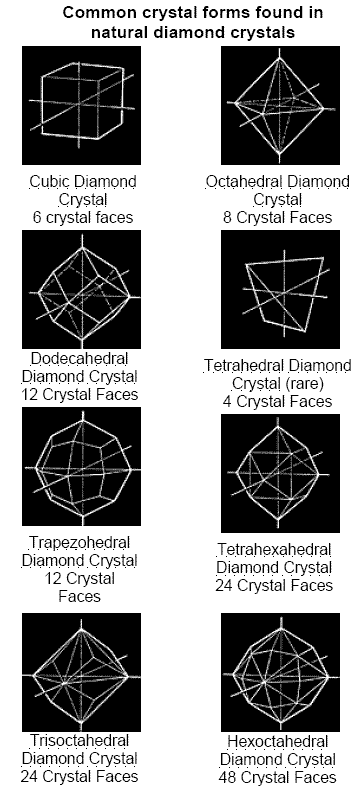
Crystallography: Isometric, commonly octahedral {111}, but also may be found as cubes {001}, or dodecahedra {110}. The faces are commonly curved. Twins on the {111} (spinel law) are common. The radial or cryptocrystalline aggregates of bort, a variety of diamond, cause it to have rounded forms or a rough exterior. Bort is also used to describe diamonds that are flawed or badly colored (Klein, 2002).
Diamond has perhaps the most fascinating crystal morphology of any mineral. Diamonds crystallize as octhedrons, cubes, or dodecahedrons and commonly exhibit habits that contain two or more of these forms. Although we find diamonds at the Earth’s surface, they form deep within the Earth. They have a rapid and violent journey from their birthplace in the mantle to the volcanoes in which they occur. Along the way the pressure that allowed them to form is rapidly removed (Kirkley, 1998). They then become unstable and begin to dissolve, leaving the surface of many diamonds etched or pitted and their overall shape occasionally rounded. Etched diamonds can also exhibit a cross figure on its faces. Whether through growth or etching, another crystal form is sometimes observed: the hexoctahedron (six faces on each of the octahedral faces). Other interesting features are the etched pits found on the faces of diamonds. Since these are frequently triangular, these etched pits are called trigons. The face of an octahedron is a triangle. Trigons found on octahedrons are oriented such that the point of its triangle is pointed in the opposite direction of point of the triangle of the octahedral face. Figure 1 shows an octahedral diamond crystal modified by a hexoctahedron (evidenced by the fact that the triangular octahedron face really has six sides). Furthermore, this drawing shows etched trigons. Notice that trigon and octahedron triangles point in opposite directions (Sunagawa, 2005).
Physical properties: Diamond is the hardest known mineral, H=10, and a specific gravity of 3.51. Diamonds luster is adamantine and uncut crystals have a greasy appearance. High refractive index (2.42) and dispersion. Color is pale yellow or colorless, but may be found in many other colors. Diamond has perfect octahedral cleavage {111}.
Chemistry: Diamond is pure carbon. General Electric first synthesized diamond in 1955 at pressures of 600,000 to 1,500,000 psi and temperatures of 750 to 2,750 degrees C. In 1967, GE also produced a hexagonal polymorph of diamond that was later discovered in the Canyon Diablo meteorite in Arizona. This hexagonal polymorph is lonsdaleite, which has also been found in the Goalpara meteorite and in sands in various parts of Russia (Bernard and Hyr?l, 2004).
Occurrence: The most common geologic setting of diamonds is in diamond pipes, small diameter volcanic features. The volcanic host rock is kimberlite, whose magma type is generated deep within the earth’s mantle. This rock weathers to a yellow rock near the surface (yellow ground), which gives way to a darker fresh rock at depth (blue ground). Diamonds liberated by weathering also accumulate in alluvial deposits (Klein, 2002). Diamonds are found, but not formed, in kimberlite. In nature, diamond forms at temperatures in excess of 1200 C (2200 F) and at pressures 44,000 times that of atmospheric pressure. These conditions are found only at depths of 100 miles below the surface of the Earth. The two main rock types in which diamond is formed are harzburgite and eclogite, both quite rare igneous rock types. Harzburgite is composed of olivine, bronzite (an orthopyroxene), and some pyrope (chrome- and magnesium-rich varieties). Harzburgitic diamonds contain inclusions of olivine, broinzite, and pyrope. Harzburgitic diamonds are more than 3 billion years old. Eclogite is a high pressure metamorphic rock composed of garnet (almandine-pyrope variety) and omphacite (a sodium-rich clinopyroxene). Diamond is so abundant in some of these eclogites that is may comprise ten percent of the rock! An extremely fascinating mineral inclusion that occurs in eclogite is diamond inclusions in garnet. They are microscopic, but very unusual when you consider how they came to be. Kimberlite is then derived from melt of eclogite or harzburgite and will occasionally contain inclusions (xenoliths) of these diamond source rocks (Kirkley, 1998).
The most productive diamond producing regions are southern Africa, Siberia, India, and Brazil. There are other minor occurrences in the United States: Murfeesboro, Arkansas; and Colorado and Wyoming (Klein, 2002).
Uses: Diamonds extreme hardness, luster, and dispersion together with its rarity make it one of the most sought after gemstones. Its hardness also makes diamond useful in industry for abrasives and for saws and drills (Klein, 2002).
Bernard, Jan H., and Hyr?l, Jaroslav, 2004, Minerals and Their Localities. Granit Publishing House, Czech Republic, 807 p.
Blackburn, W.H., and Dennen, W.H., 1997, Encyclopedia of Mineral Names. The Canadian Mineralogist Special Publication 1, Mineralogical Association of Canada, 360 p. Field, J.E., 1979, The Properties of Diamond. Academic Press, Ltd., London, 674 p.
Harlow, George E., 1998, “What is a Diamond?” in Harlow, George E., The Nature of Diamonds. Cambridge University Press in association with the American Museum of Natural History, New York, p. 1-22.
Kirkley, Melissa B., 1998, “The Origin of Diamonds: Earth Processes”. in Harlow, George E., The Nature of Diamonds. Cambridge University Press in association with the American Museum of Natural History, New York, p. 48-65.
Klein, C., 2002, The 22nd edition of the Manual of Mineral Science (after J.D. Dana). John Wiley & Sons, Inc., New York, 681 p.641 p., CD-ROM.
Moore, Thomas, 2004, “A Collection of Diamond Crystals with Notes on Science, History, and Worldwide Localities of Diamonds”. Mineralogical Record, vol. 35, no. 1, p. 9-30 and 53-54.
Orlov, Yu. L. (ed.), 1973, The Mineralogy of the Diamond.John Wiley & Sons, New York, 235 p.
Seal, Michael, 1965, “Structure in Diamonds as Revealed by Etching”. American Mineralogist, vol. 50, p. 105-123. Sunagawa, Ichiro, 2005, Crystals: Growth, Morphology, and Perfection. Cambridge University Press, Cambridge, 295 p.
Diamond Crystal Diagrams from Goldschmidt's Atlas der Krystalformen
Source: http://www.johnbetts-fineminerals.com/jhbnyc/diamdiag.htm
Photo courtesy of Julian C. Gray
Diamond octahedron (2.5 mm wide)
Udachnoe Kimberlite Pipe, Sakha, Russia
Julian C. Gray, Specimen (ex. Bill Dameron)

Copyright © Georgia Mineral Society, Inc.
